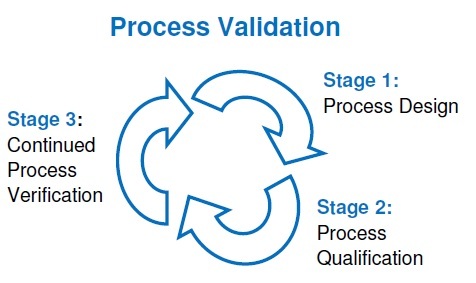
Pharmaceutical companies in Vietnam pursue EU-GMP
EU GMP is a standard of good manufacturing practice for medicines issued by the European Medicines Agency (EMA), covering all principles and standards to control activities or problems occurring in pharmaceutical manufacturing facilities in order to ensure the safety, efficacy, stability, and uniformity of each batch of products. Advantages of