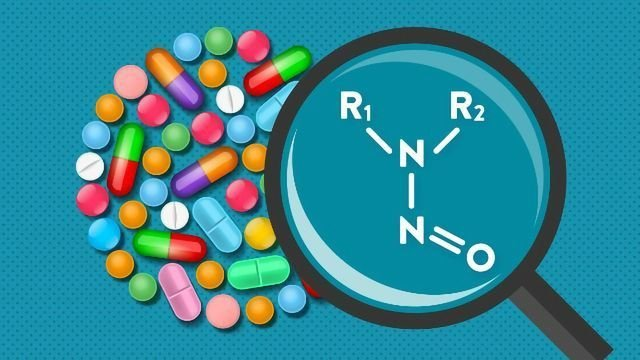
What do we know about N-nitroso compounds (NOCs)?
NOCs were first discovered about 40 years ago when they were found to be present in processed foods treated with sodium nitrite, such as bacon, cheese, cured meats, and fish. The most common NOCs are N-nitrosodimethylamine (NDMA) and N-nitrosodiethylamine (NDEA) nitrosamines.
Both laboratory and animal studies demonstrate that NOCs can cause cancer in many tissues of the body. This risk becomes higher with prolonged exposure to nitrosamine impurities. The detection and identification of nitrosamine impurities in several commonly prescribed medicines, such as ARBs, antibiotics, antacids, and antidiabetics, led to the recall of these products worldwide, including for products of multinational pharmaceutical companies
How is nitrosamine formed in drug products?
Nitrosamine is formed from the reaction between nitrite and secondary amine under acidic conditions. Some risk factors should be considered including:
- The use of sodium nitrite or other nitrosating agents during drug production
- The presence of nitrosamine precursors in raw materials, excipients, packaging
- The presence of highly reactive elements in APIs, raw materials, intermediate products
- Using the synthetic pathway or specific manufacturing process conditions that are acid
- Exposing to nitrogen oxides (NOx) during the production and storage stages
What can the drugmakers do to overcome the risk of nitrosamine contamination in their drug products?
- Review existing drugs’ formulations and manufacturing processes to prevent nitrosamine formation
- Add specific antioxidants such as ascorbic acid or alpha-tocopherol to formulations as a nitrosamine formation inhibitor (FDA recommended)
REFERENCE:
Expert view: 5 things you need to know about nitrosamines in drug formulation