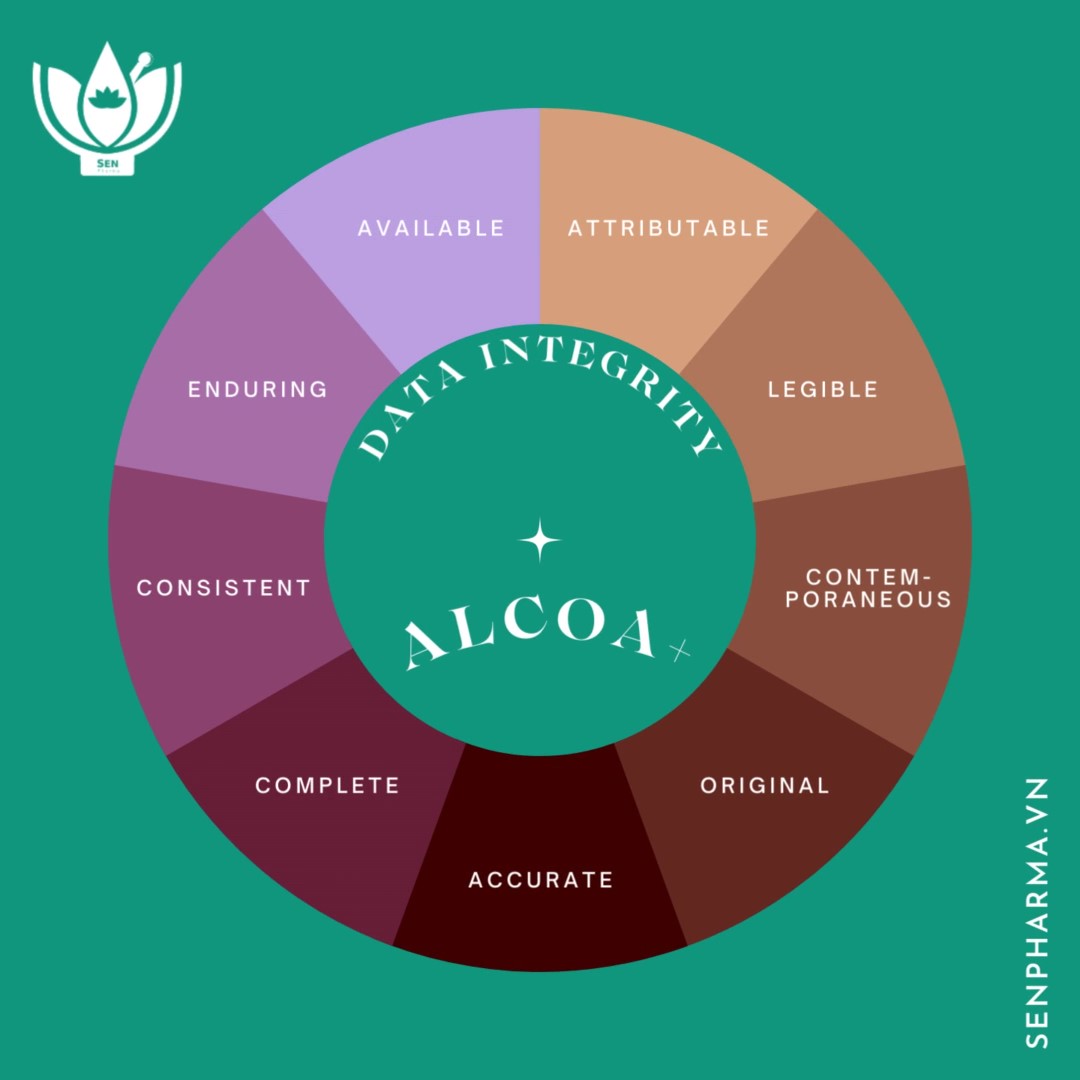
In 2022, many pharmaceutical factories were entangled in data integrity (DI) issues during inspections by authorities. In some severe cases, such as the factory of Centrient Pharmaceuticals (specializing in Dutch antibiotics) in Punjab, India, received an FDA warning letter in December 2022.
Which errors did occur?
- GMP documentation at the factory is managed improperly.
For example, investigators discovered numerous logbooks, forms, and incompletely filled sample requests stored in a temporary uncontrolled storage room near the microbiology laboratory. Moreover, the investigators found a document shredder labeled “Emergency use” containing many shredded documents in this room. Those docs included a great deal of important information, like relative humidity, temperature, and handwritten data.
- The computer system is controlled inadequately.
For example, a microbiologist’s handbook has multiple user accounts and system login passwords. This deviation violates the data attributability in the set of DI regulations.
This factory has been required to give a full explanation. Otherwise, the drug export to the US market will be stopped. This problem cost the company lots of money and severely damaged the business.
This matter causes great concern to the FDA
Recently, the FDA has sent out many warning letters to manufacturers with factories in India or even in the US.
- In December 2022: An FDA warning letter was sent to a factory in Goa of a large generic drug company Glenmark. Among many errors pointed out in the letter, there was incomplete information relating to a production batch, which violated the data’s completeness.
- In November 2022: The Kari Gran plant in Seattle received a warning letter from the FDA. The letter highlighted errors in data completeness regarding the production batch and quality control process. The warning letter also emphasized that the current factory does not have a quality system yet to fully guarantee the data accuracy and integrity requirements to bring good quality, safe and effective medical products to market.
Due to the seriousness of data integrity errors, Vietnamese pharma companies need to pay more attention to these problems to avoid consequences such as product recalls, fines, or even suspension.
NGUỒN THAM KHẢO:
- WARNING LETTER – Centrient Pharmaceuticals India Private Limited
- WARNING LETTER – Glenmark Pharmaceuticals Limited
- WARNING LETTER – Kari Gran Inc.