1. Overview
In 1997, the Nanogen Biopharmaceutical Technology Limited Liability Company was established with the mission to develop and produce safe and effective biological therapies at reasonable prices. As of the end of 2020, Nanogen’s total assets exceeded 1,400 billion VND, with a charter capital of over 800 billion VND.
2. Research and Production Activities
Presently, Nanogen operates four manufacturing plants and one research center in Ho Chi Minh City and Lam Dong province. The company’s manufacturing facilities have achieved TGA-GMP standards (Australia) and WHO-GMP standards.
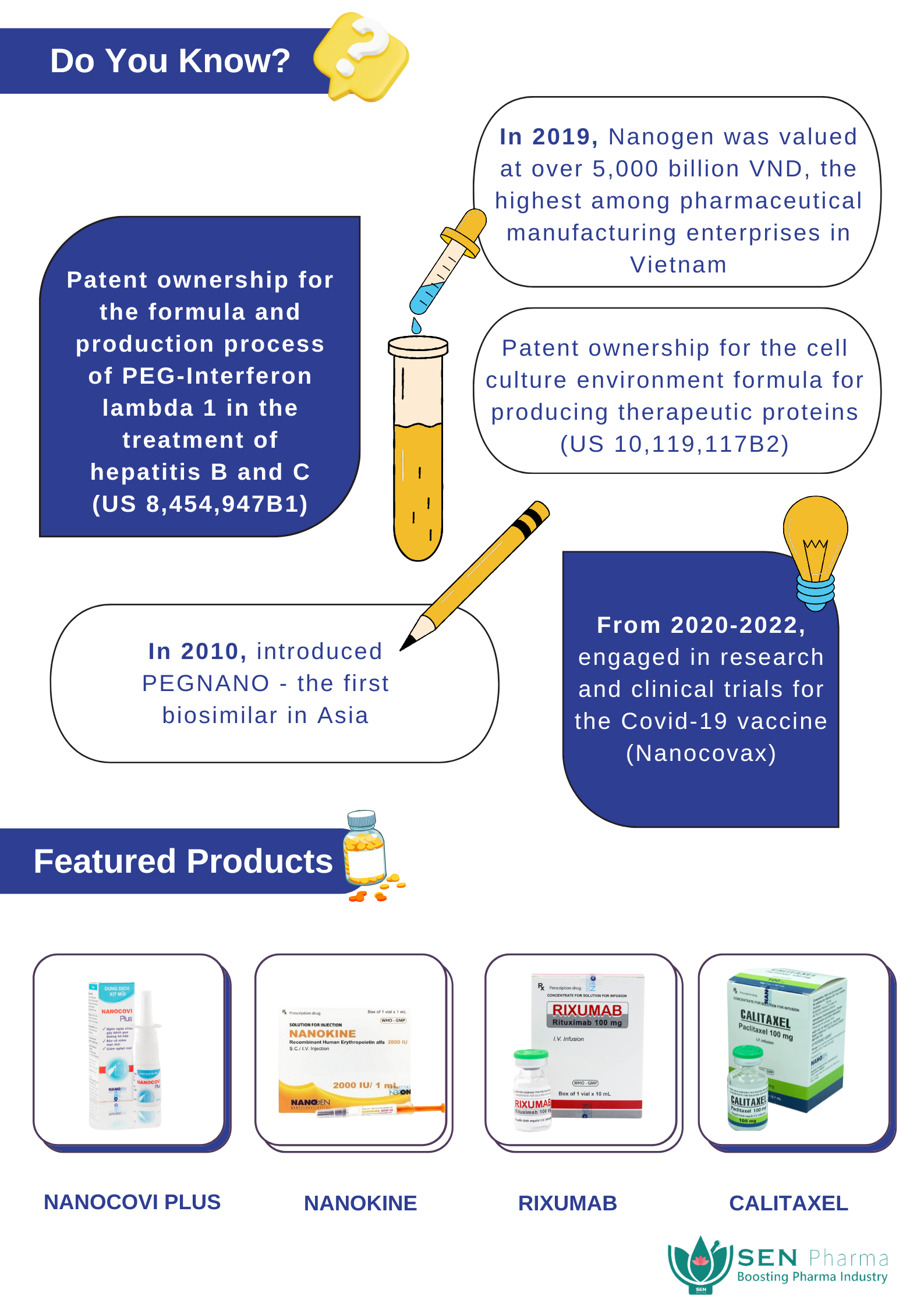
Since the construction of the biopharmaceutical manufacturing plants in 2009, the Nanogen team has continuously endeavored in research, innovation, and clinical testing of safe and effective biological products. Particularly noteworthy is the introduction of the biosimilar product PEGNANO in 2010, the first of its kind in Asia. Additionally, Nanogen has obtained patents in the United States and distributes its products to 15 countries across Asia, Europe, Africa, and the Americas.
The prominent product lines of the company include treatments for hepatitis B, hepatitis C, anemia, and various cancers such as:
- PEGNANO: treatment of chronic hepatitis B virus infection and chronic hepatitis C virus infection.
- FACEPTOR: breast cancer treatment..
- MIRACEL: for breast cancer, cervical cancer, gastric cancer, prostate cancer, and non-small cell lung cancer, among others…
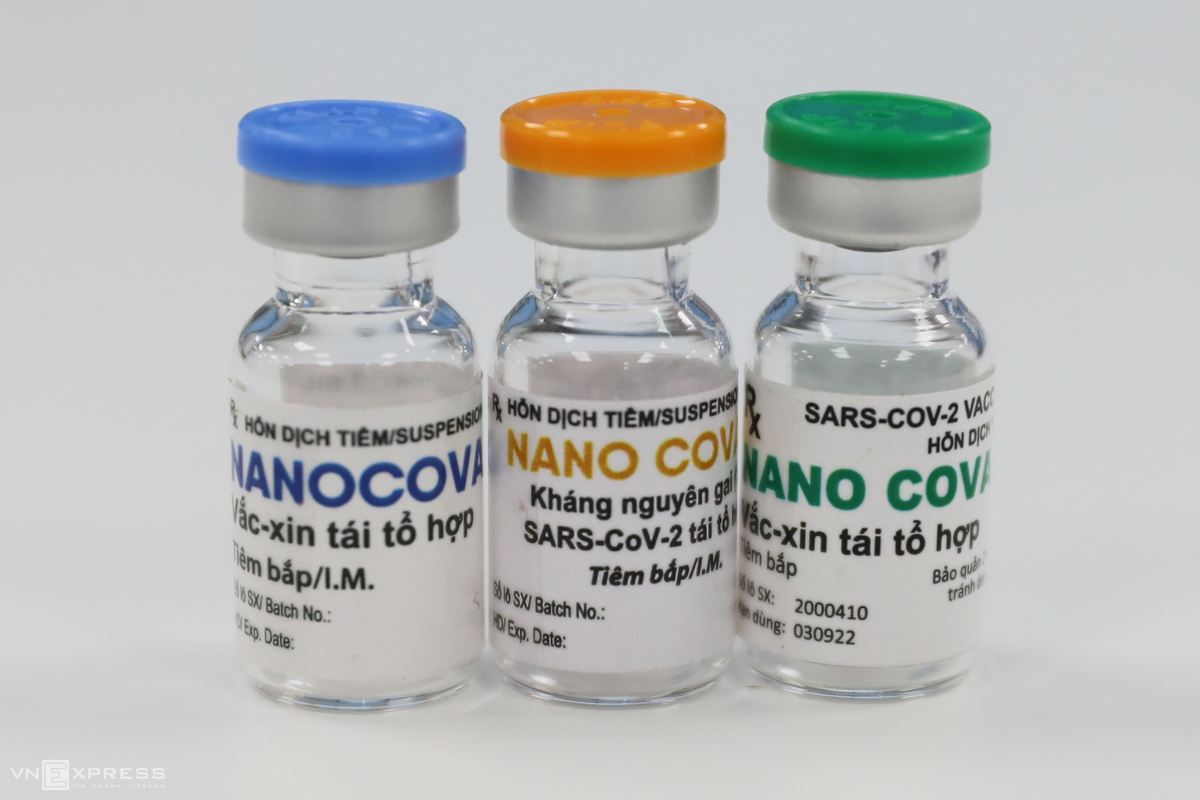
During the outbreak of the Covid-19 pandemic, Nanogen emerged as a pioneering pharmaceutical company in Vietnam, actively participating in the development of the Covid-19 preventive vaccine. Nanogen has progressed its research and development efforts to Phase 3 clinical trials for the NANO COVAX vaccine. This marks a new milestone in the development of biopharmaceutical technology in Vietnam, potentially attracting foreign investors and businesses to invest in the country.
REFERENCES:
CÔNG TY CỔ PHẦN CÔNG NGHỆ SINH HỌC DƯỢC NANOGEN