The drug liquid products have been one of the most popular dosage forms for centuries. The packages play a crucial role in quality assurance and business manners. Blow-fill-seal (BFS) technology emerged as an advanced solution for liquid drugs, especially aseptic products. This technology has been used for more than 30 years and is accepted by any regulatory administration, such as FDA.
The BFS is a continuous process in which polymers are heated to 200 celsius, followed by blowing into the mold to form containers. Then, the liquid products are continuously filled into the formed containers and removed from the mold. All procedures are conducted in a close area without human intervention.
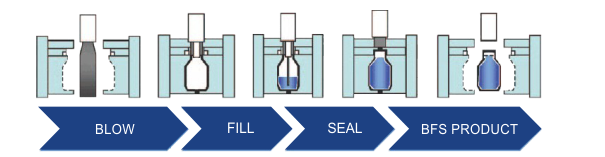
The polymers commonly used for BFS include low-density polyethylene (LDPE), high-density polyethylene (HDPE), and polypropylene (PP). LDPE is suitable for large molecules of drugs or vaccines due to the ease of forming the appropriate shape. While PP, which is durable to sterile under high temperatures, is used for aseptic products.
What are the advantages of blow-fill-seal technology?
The significant advantage of this technology is the reduction of contamination related to people. Many reports proposed that the contamination with this system is below 0,1%. It takes 15 seconds to complete a product. For parenteral products or infusion, the BFS eliminates the drawback of traditional glass containers, such as breakage or glass-particulate contamination. Moreover, the single dose provided by this system reduces the wastage of multi-dose glass type and the loss during the filling process. Because of the flexibility of the size of formed containers, the BFS can create large-volume containers for infusion products. Besides, BFS provides a diversity of shapes of packages for commercial purposes. BFS is also an advanced solution for aseptic products which can not withstand terminal sterilization, including biological products. The BFS is easy to rapidly scale production for surge capacity because of fewer package components than traditional packages and a consolidated materials supply chain that helps decrease reliance on external sources
What are the challenges?
Because of the new packaging material application, stability studies are recommended. The compatibility of formulation and polymer should be taken into consideration, including evaluating the extractable and leachable of polymer into product contains. Biomolecules can be adsorped by polymers which may reduce the effectiveness of products. The gas and water vapor permeation through the wall of the container also is evaluated through the stability studies. In addition, with thermal-sensitive products such as biologics, the employed temperature during the process is crucial. A high temperature is needed to form containers, and they should be kept warm throughout the process to ensure the final sealing. The cold BFS can solve this problem.
REFERENCES:
- Improving Sterility Using Blow-Fill-Seal Technology
- Considering Blow-Fill-Seal for Biologic Drugs
- Blow-fill-seal Technology Advances in Aseptic Filling Applications




