The below video will summarize risk assessment methods for nitrosamines in drug products. Since 2018, many drug batches have been recalled because of nitrosamine impurities above acceptable levels.
What are nitrosamines?
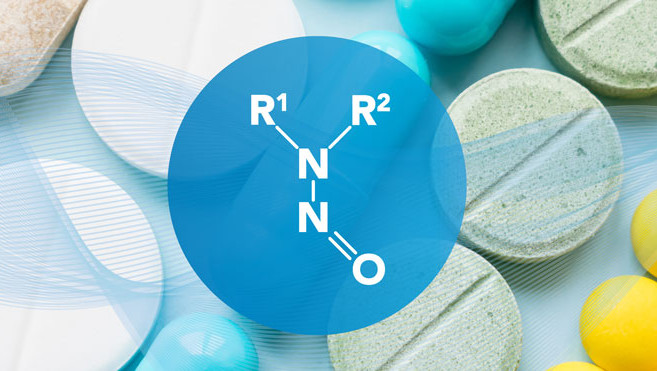
The chemical structure of nitrosamines contains an amine bonding with a nitroso group, which is formed by a reaction between secondary or tertiary amines with nitrosating agents.
Additionally, nitrosamines are known as mutagenic agents, which increase the risk of developing many types of cancer.
It is worth noting that:
- The risk of cancer is estimated to be about 33% worldwide.
- The recalled drugs are not our only source of exposure to nitrosamines. We are currently able to add a low level of nitrosamines day by day through food, water, air, and medicine, resulting in an increase in cancer incidence of at least 0.001 to 0.01%
- The safe limit for nitrosamines in drugs is lower than what people are exposed to from other sources, therefore it is a negligible increase in cancer risk (less than 0.001%).
- In some cases, drug recalls are due to nitrosamine levels exceeding the acceptable threshold, even if levels are just slightly above the acceptable threshold.
If in many cases, the risk is very low, why is it necessary to withdraw the drug from the market?
Although the concentration or risk of cancer incidence of impurities (particularly nitrosamines) in pharmaceutical products is at lower levels compared to other daily exposures, regulatory authorities always put effort into eliminating avoidable harm in drugs.
In September 2019, the European Medicines Agency (EMA) determined that pharmaceutical companies must evaluate all their synthetic drug products for the risk of nitrosamines formation and contamination.
Bài viết liên quan
REFERENCES:
- EMA/217823/2019 Assessment report. v. 31, n. February, p. 1–41, 2019.
- FDA Questions and Answers: NDMA impurities in ranitidine (commonly known as Zantac).
- ICH M7 (R1). Assessment and Control of DNA Reactive (Mutagenic) Impurities in Pharmaceuticals to Limit Potential Carcinogenic Risk, 2017.
- LITEPLO, R. G.; MEEK, M. E.; WINDLE, W. WHO Concise International Chemical Assessment Document 38: N-nitrosodimethylamine.
- IPCS Concise International Chemical Assessment Documents, n. 38, 2002.
- SNODIN, D. J.; ELDER, D. P. Short commentary on NDMA (N-nitrosodimethylamine) contamination of valsartan products.
- Regulatory Toxicology and Pharmacology, v. 103, n. April, p. 325–329, 2019.