Empagliflozin is an SGLT2 inhibitor currently used to treat type 2 diabetes under the brand name Jardiance®. In addition to its glycemic control, empagliflozin has been proven effective in patients with heart failure and chronic renal failure, opening a promising future for generic drugmakers when Jardiance® exclusivity ends.
This article by SEN Pharma on empagliflozin and Jardiance® will provide highly useful information about:
- History and mechanism of SGLT2 inhibitors
- Development timeline, and advantages of empagliflozin
- Sales of Jardiance®
- The key development points for generic drug makers
SEN Pharma is proud to be a partner of Unipharm, a leading raw material distributor in China that owns a source of empagliflozin, complete with documents and reasonable prices. With close ties to more than 100 raw material factories in China and the distribution of more than 700 APIs, Unipharm is the best place for pharmaceutical factories in Vietnam to get raw materials, not just for empagliflozin but also for many other potential active substances.
The history and the mechanism of SGLT2 inhibitors
The history of SGLT2 inhibitors
The first SGLT2 inhibitor active component, phlorizin, was discovered in 1835 by a French scientist named C. Petersen, who extracted it from the root bark of several fruit trees such as pears and apples. Phlorizin, however, was initially utilized to treat malaria. It was not until 1886 that the effectiveness of phlorizin in lowering blood glucose was discovered by a German professor of pharmacy named Von Mering. [1]
However, because of limited absorption in the gastrointestinal system, phlorizin was gradually replaced by later synthetic active substances such as canagliflozin, dapagliflozin, and empagliflozin. Empagliflozin, in particular, outperforms phlorizin in terms of potency, longer half-life, and significantly improved bioavailability [1].
The mechanism of SGLT2 inhibitors
During the first half of the twentieth century, most people assumed that sugar molecules would be totally reabsorbed by the renal tubules after being filtered by the glomerulus. However, scientists progressively realized that this reabsorption needed active transport by sodium-glucose co-transporters (SLGTs) in renal tubular cells during the 1960s [1], [2]. In which up to 90% of the sugar discharged in urine is reabsorbable through the SGLT2 transporter channel.
With a better understanding of SGLT transporter activity in the body since 1995, prominent pharmaceutical companies have been racing to develop potential SGLT2 selective inhibitors. Invokana® (canagliflozin) from Janssen was the first medicine in the SGLT2 inhibitor class to be introduced in 2013 [3]. Farxiga® (dapagliflozin) and Jardiance® (empagliflozin) were introduced in 2014, respectively, [4], [5].
In addition to their primary role in blood sugar regulation, SGLT2 inhibitors also cause weight loss and blood pressure regulation. This is also the optimal choice to combine with metformin in cases of poor blood sugar control with weight gain and high blood pressure. [2]
In addition, SGLT2 inhibitors have been proven to lower cardiovascular events and fatalities in type 2 diabetic patients. Furthermore, people with heart failure who are not diabetic have also been reported to benefit from cardiac advantages. Scientific evidence has changed heart failure guidelines around the world as a diabetes drug is brought up to par with traditional heart failure medications. [2], [6]
The development timeline and advantages of empagliflozin
The development timeline of Jardiance®
On January 11, 2011, Boehringer Ingelheium and Eli Lilly announced that they were in the final stages of developing a diabetes drug portfolio, including two potential active ingredients at the time, linagliptin and BI10773 – later named empagliflozin [7]. In fact, the clinical results of empagliflozin were impressive by reducing HbA1c by about 0.62% at a 10 mg dose and 0.66% at a 25 mg dose [8].
On April 2, 2013, the first SGLT2 inhibitor approved by the FDA, however, was Invokana® (canagliflozin) by Jassen, since the approval of Jardiance was refused by the FDA until issues at the product manufacturing facility of Eli Lilly are resolved. [7]
On August 1, 2014, Jardiance® was officially approved by the FDA after the drug was approved in Europe by the EMA on May 23, 2014. On December 2, 2016, the FDA approved a new indication of Jardiance® to reduce the risk of cardiovascular death in patients with diabetes. This is the first diabetes drug to be approved for this indication.
On February 24, 2022, the FDA officially recognized the effectiveness of Jardiance® in reducing the risk of death and hospitalization from heart failure in patients with heart failure.
Boehringer Ingelheium and Eli Lilly also combine empagliflozin with other antidiabetic agents such as linagliptin (brand name Glyxambi® – launched January 30, 2015) and metformin (brand names Synjardy® and Synjardy®). XR – released August 26, 2015) [9], [10].
Advantages of empagliflozin
Empagliflozin has the highest sensitivity to SGLT2 when compared to other SGLT2 inhibitors, blocking SGLT2 2500 times more than SGLT1, a transport channel that reabsorbs 10% of the excreted sugar. [8]
Empagliflozin decreased the progression of chronic kidney disease, according to recent research released in November 2022. In particular, it lowers mortality risks from heart disease and chronic renal disease by up to 28%. These findings came from clinical research involving both diabetic and non-diabetic people. After dapagliflozin, which is marketed under the trade name Farxiga®, empagliflozin is the next active component to be licensed for the treatment of chronic renal failure. [11]
Sales of Jardiance®
Global revenue for Jardiance® has increased annually since its 2014 introduction. Particularly from 2020 until the present, when Jardiance® and other SGLT2 inhibitors have profited from the COVID-19 pandemic as diabetics are more vulnerable to COVID-19 due to their weaker immune systems [12].
For the first time, Jardiance® generated more than $6 billion in revenue in 2021, making up 56.2% of the total market revenue for SGLT2 inhibitors. [7], [12]
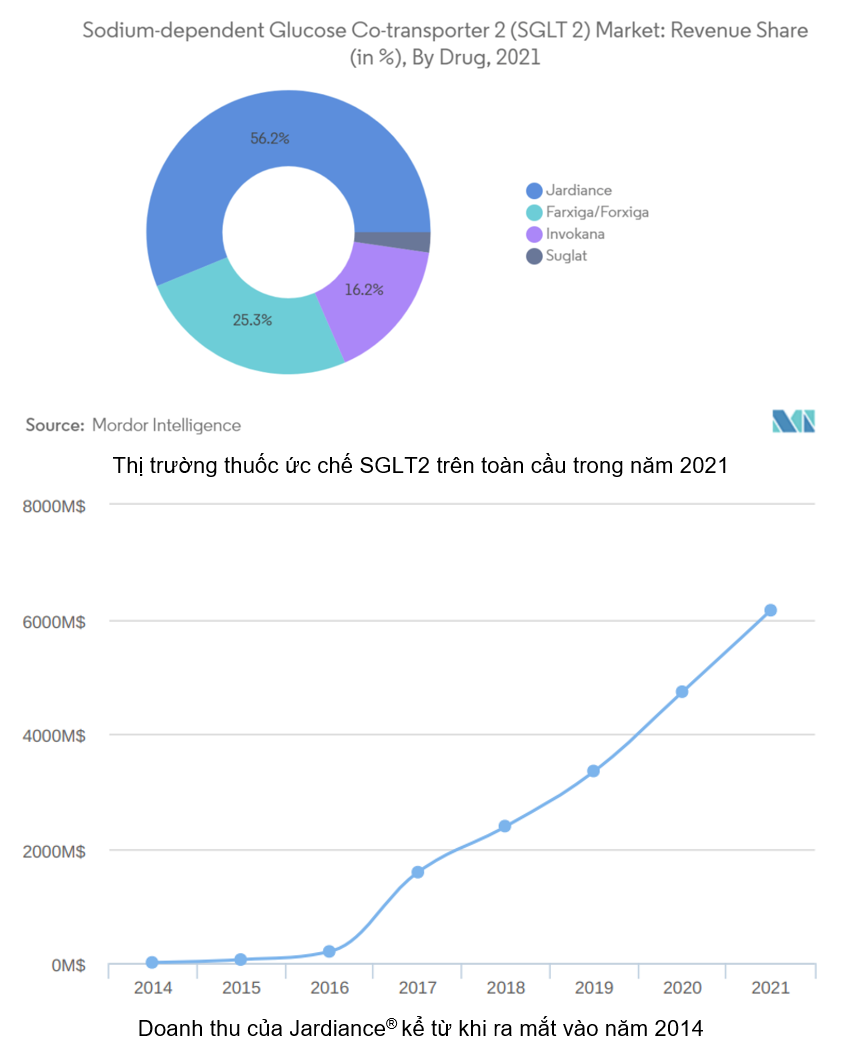
However, Jardiance® sales are likely to increase in the coming years due to its effectiveness in the treatment of heart failure and chronic renal failure. As a result, Jardiance® competes not only with other SGLT2 inhibitors like Farxiga® (dapagliflozin), but also with particular medications for heart failure like Verquvo® (vericiguat) [12].
Jardiance® is currently available in Vietnam for VND 26,333 (25 mg) and VND 23,072 (10 mg) [13].
The key development points of empagliflozin
Boehringer Ingelheium and Eli Lilly jointly own many patents related to the active ingredients and formulations of Jardiance®, including:
- Active ingredient patent: US7713938B2 (expiration date 15/04/2027 [14])
- Active ingredient patent: US 7579449B2 (expiry date 01/08/2028 [14])
- Pharmaceuticals patent: WO2010092126A1
On SEN Pharma’s website, readers can find information about Jardiance® compositions and manufacturing processes in the Library – Brandname section.
Apparently, to prepare for formula research, stability monitoring, pilot batch production, and regulatory submissions, generic drug companies in Vietnam have started searching for empagliflozin to meet domestic treatment needs as soon as the active ingredient patent expires (around mid-2027).
In addition, the optimal particle size range for empagliflozin was highlighted in patent WO2010092126A1, because it influences not only the phases of the production process (such as stickiness) but also the solubility and bioavailability of the completed product [15]. This is critical for the present trend of bioequivalent drug research and manufacture in Vietnam, especially since Circular 07/2022/TT-BYT went into effect in November.
SEN Pharma is proud to be a partner of Unipharm, a leading raw material distributor in China that owns a source of empagliflozin, complete with documents and reasonable prices. With close ties to more than 100 raw material factories in China and the distribution of more than 700 APIs, Unipharm is the best place for pharmaceutical factories in Vietnam to get raw materials, not just for empagliflozin but also for many other potential active substances.
REFERENCES:
- [1] E. Braunwald, “SGLT2 inhibitors: the statins of the 21 st century”
- [2] “Lợi ích ngoài kiểm soát đường huyết của nhóm thuốc ức chế SGLT2 | Vinmec.”
- [3] “Invokana: Uses, Dosage & Side Effects – Drugs.com.”
- [4] “Jardiance: Uses, Dosage, Side Effects & Warnings – Drugs.com.”
- [5] “Farxiga Uses, Dosage, Side Effects & Warnings – Drugs.com.”
- [6] “Thuốc ức chế SGLT2: Trụ cột mới trong phác đồ điều trị suy tim | Tim mạch học.”
- [7] “Home – PharmaCircle.”
- [8] G. Chawla and K. K. Chaudhary, “A complete review of empagliflozin: Most specific and potent SGLT2 inhibitor used for the treatment of type 2 diabetes mellitus,” Diabetes Metab Syndr, vol. 13, no. 3, pp. 2001–2008, May 2019, doi: 10.1016/J.DSX.2019.04.035.
- [9] “Glyxambi (empagliflozin and linagliptin) FDA Approval History – Drugs.com.”
- [10] “Synjardy (empagliflozin and metformin) FDA Approval History – Drugs.com.”
- [11] “Lilly, Boehringer say Jardiance slows kidney disease progression in trial | Reuters.”
- [12] “Sodium-Dependent Glucose Co-transporter 2 (SGLT2) Market 2022 – 27.”
- [13] “Website chính thức của Cục Quản Lý dược, Bộ Y Tế.”
- [14] “Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations.”
- [15] “WO2010092126A1 – Pharmaceutical composition comprising glucopyranosyl diphenylmethane derivatives, pharmaceutical dosage form thereof, process for their preparation and uses thereof for improved glycemic control in a patient – Google Patents.”