Chuyên mục: Quality
Webinar “Quality Management System: Common Issues And Better Solutions”
Many manufacturers in Vietnam face numerous obstacles while developing and improving their pharmaceutical quality systems,
Pharmaceutical companies in Vietnam pursue EU-GMP
EU GMP is a standard of good manufacturing practice for medicines issued by the European
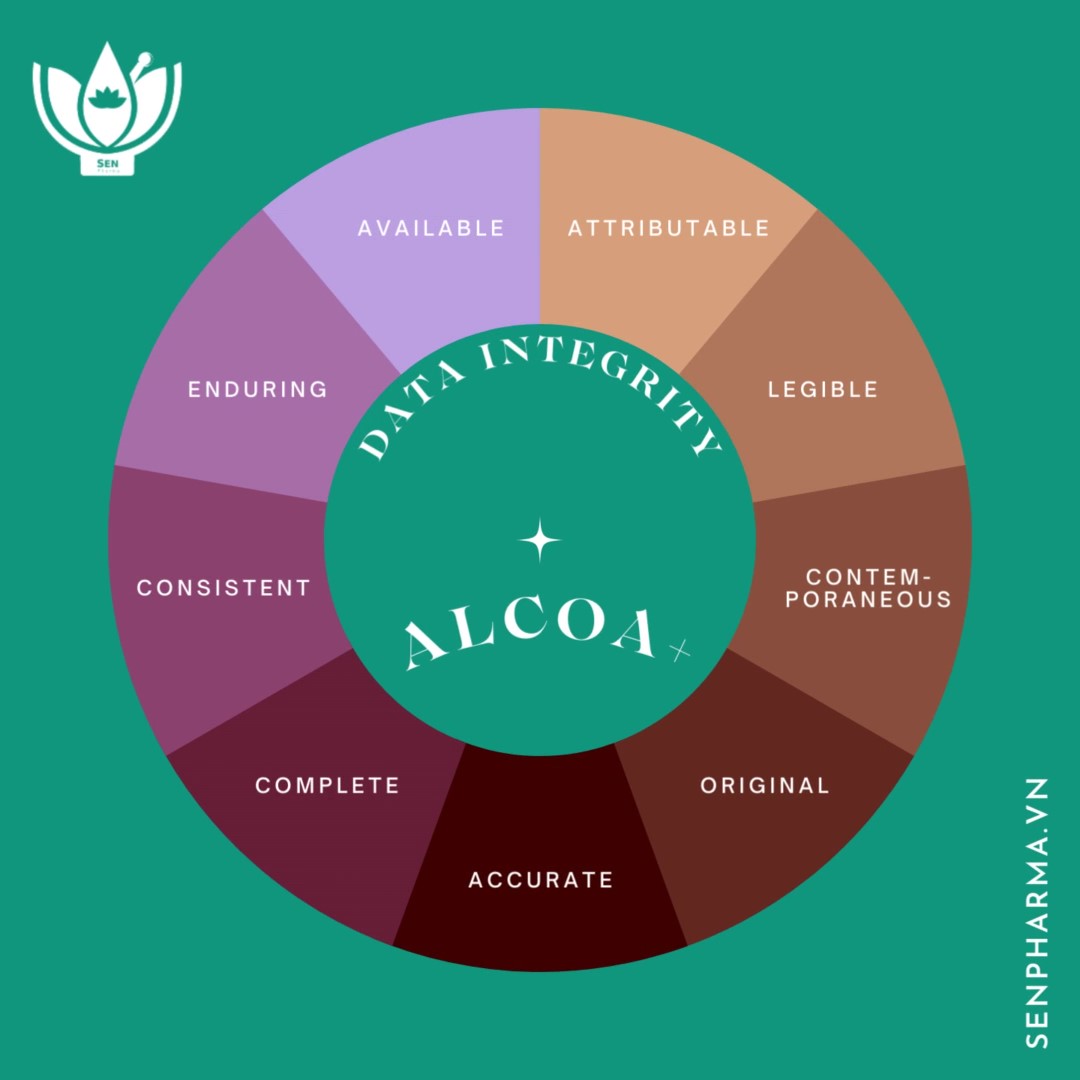
Data Integrity – The achilles heel in pharmaceutical factories
In 2022, many pharmaceutical factories were entangled in data integrity (DI) issues during inspections by
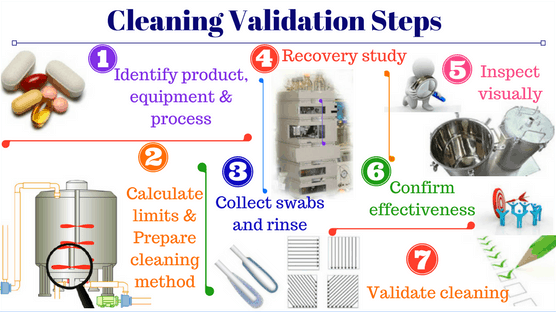
Cleaning validation in pharmaceutical manufacturing
Medicine is of significance for human well-being, thus, the pharmaceutical production process must adhere strictly
Recurrence of quality defects – A serious issue
With the mission of promoting the development of the pharmaceutical industry, SEN Pharma always encourages
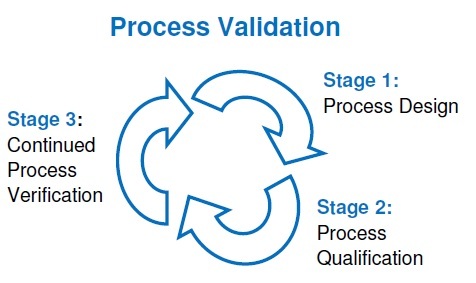
Introduction of process validation
What is the process validation? Process validation is the collection of data and documents that
Does the entire batch have to be destroyed if it does not meet pre-defined specifications?
Even if the process is validated, the equipment is qualified, the in-process parameters are controlled,
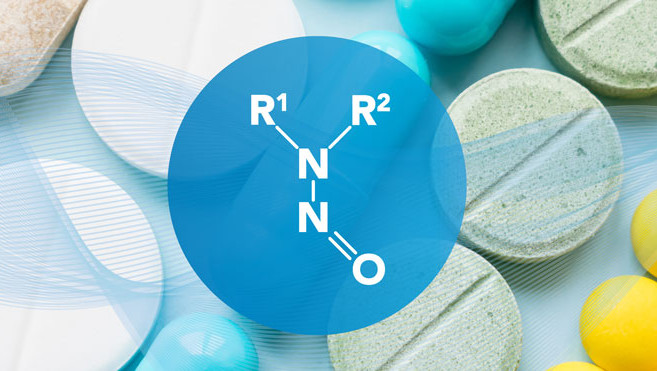
The need for Nitrosamine risk assessment?
The below video will summarize risk assessment methods for nitrosamines in drug products. Since 2018,