Chuyên mục: Regulatory affair
The Federal Trade Commission warns drugmakers against improper patent filings
The Federal Trade Commission (FTC) is warning drug companies against improper patent filings, saying that
Circular No. 16/2023/TT-BYT about marketing authorization registration of contract manufactured medicine, medicine of technology transfer in Vietnam
This circular prescribes marketing authorization registration of contract manufactured medicine, medicine of technology transfer in
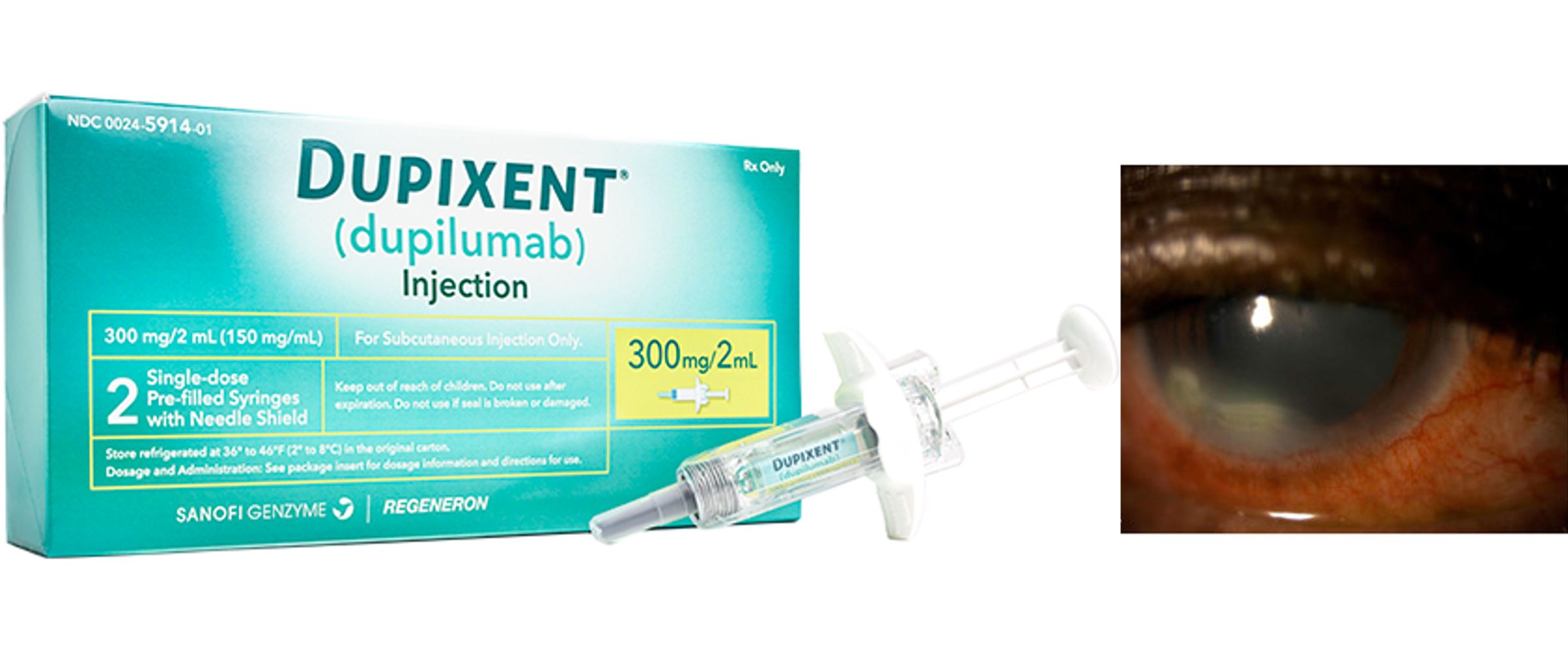
MHRA warns of new serious side effects of Dupixent®
On November 29, 2022, the Medicines and Healthcare Products Regulatory Agency (MHRA) announced new serious
A FDA-approved product
Not all products go through FDA experts’ evaluation before they are offered for sale. In
A patient instruction for use (IFU) document for human prescription drug and biological products by FDA
In July 2022, the FDA published guideline providing recommendations for developing the content and format
The top 10 highlights in Circular no. 08/2022/TT-BYT on Marketing Authorization of drugs and medicinal ingredients
On September 5th 2022, Mr. Do Xuan Tuyen, Deputy Minister of Health, promulgated the new