Fenofibrate is a class II – BCS drug that reduces triglycerides in people with hypertriglyceridemia, possibly reducing the risk of pancreatic disease (pancreatitis). Because of its poor bioavailability, fenofibrate was a challenge for manufacturers. Abbott is the maker of branded fenofibrate. This is a story of how Abbott’s fenofibrate franchise avoided generic competition.
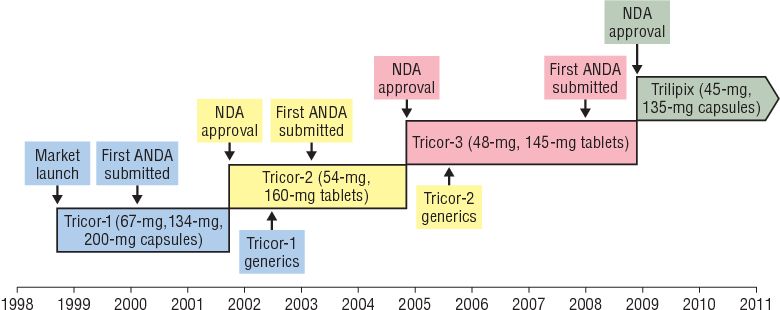
1. The story of Tricor-1: Research & Market launch
In 1980, Fournier Laboratories started developing fenofibrate and submitted a new drug application (NDA, 1984). However, FDA rejected it after a review of the cardiovascular effects.
In 1993, fenofibrate was approved for its effectiveness in decreasing triglyceride levels.
In 1998, Fournier authorized a fenofibrate patent to Abbott. Then, the drug was launched to the market with the brand name Tricor-1. Tricor-1 was designed in capsule form with three strengths: 67 mg, 134 mg, and 200 mg.
In February 2000, Novopharm (later acquired by Teva), a generic drug company, challenged Tricor-1’s patent by submitting a new drug application (ANDA). Abbott quickly responded by filing a claim for a patent infringement suit, which legally delayed the approval of this ANDA by 30 months or until the litigation was resolved. Therefore, Tricor-1 was protected from competition.
2. The story of Tricor-2: Development of the dosage form
Eighteen months after the challenge, Abbott released Tricor-2 with a successful improvement of the Tricor-1 formulation based on bioequivalence studies without any clinical trials. The new 54 mg and 160 mg tablets had the same pharmacological properties as Tricor-1.Tricor-2 accounted for 97% of fenofibrate prescriptions at that time.
During 2002 – 2003, Abbott prosecuted Teva and Ranbaxy for an ANDA application and sought to manufacture a Tricor-2 generic drug.
3. The story of Tricor-3: Diversity of content
Abbott continued to introduce Tricor-3 tablets. Similarly, Tricor-3 is bioequivalent to Tricor-1. The outstanding advantage of Tricor-3 is the convenience of usage. It can be used at any time, including the state of hunger, while Tricor-1 and Tricor-2 are administered with food.
In November 2004, Tricor-3 was approved within 70 days and rapidly dominated the market. Tricor-3 made up 96% of fenofibrate prescriptions.
4. The story of Trilipix: Derivative development
In December 2007, just before Teva submitted the ANDA for the generic drug Tricor-3, Abbott submitted the NDA for a fourth formulation called Trilipix with a capsule formulation. The highlight is that the active substance is a metabolite of fenofibrate (fenefibric acid). Trilipix helps increase HDL along with lower LDL and triglycerides. Trilipix can be taken with statins. Trilipix was granted data exclusivity for three years, which lasted until December 2011.
Thanks to this strategy, Abbott successfully extended the fenofibrate patent and dominated the market. Despite many controversial opinions, Abbott’s patent extension strategy is impressive and effective.
REFERENCES:
How Abbott’s Fenofibrate Franchise Avoided Generic Competition