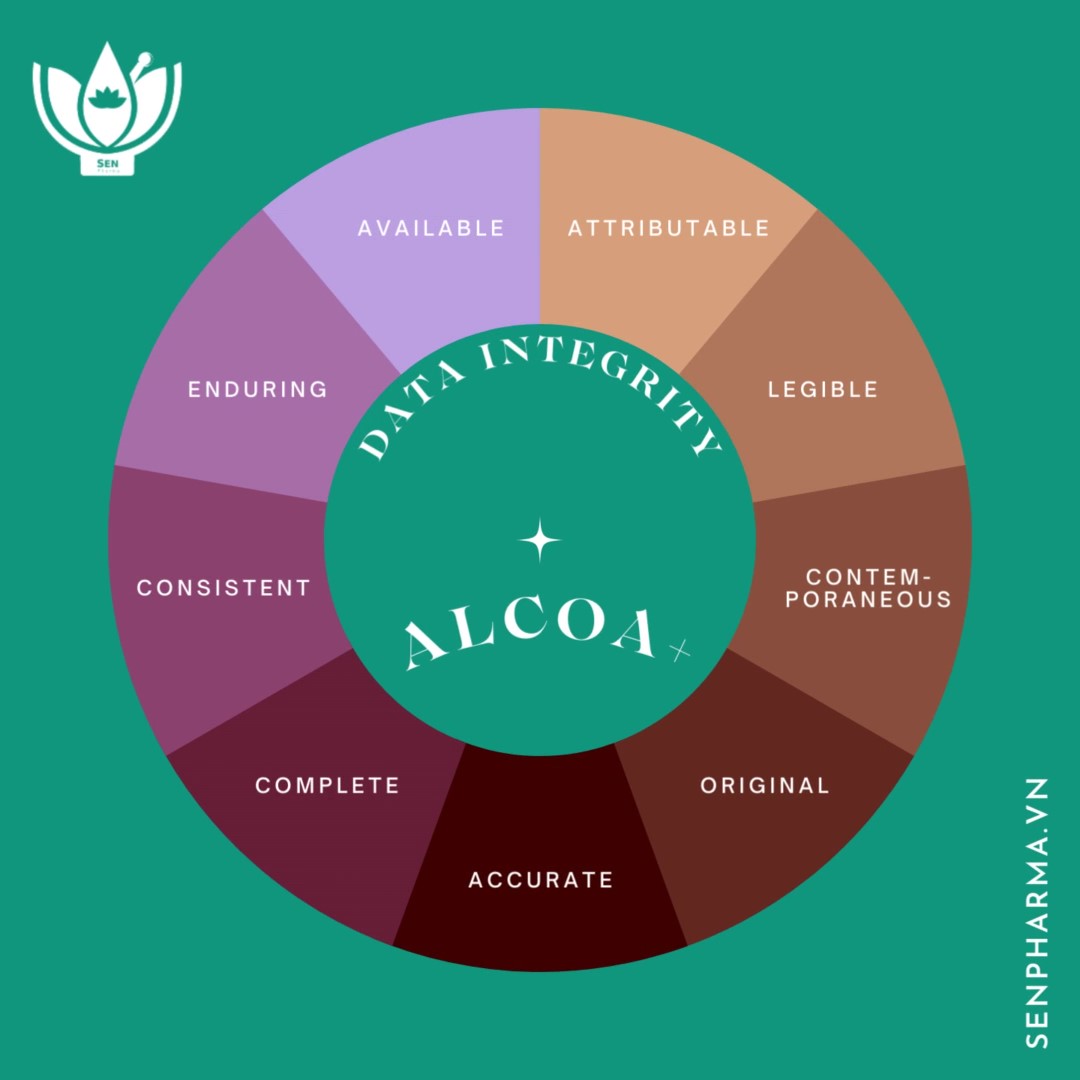
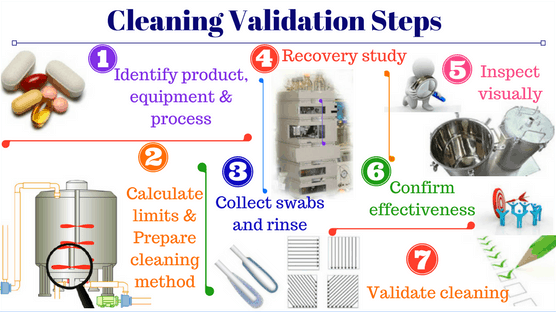
Many manufacturers in Vietnam face numerous obstacles while developing and improving their pharmaceutical quality systems, particularly when upgrading to EU-GMP standards or equivalent.
- How do we improve the quality culture?
- How can quality improvement be maintained?
- How do we properly investigate and resolve quality incidents?
Understanding these concerns, SEN Pharma invited Mr.George Nguyen, Quality Director of STADA ARZNEIMITTEL AG, to provide effective ideas to improve the quality management system at the webinar “Quality Management System: Common Issues and Better Solutions”.
Mr. George Nguyen, who has about 20 years of experience establishing quality management systems for pharmaceutical plants satisfying cGMP and EU-GMP standards in numerous countries (USA, Switzerland, Singapore, Korea, Vietnam…), will offer practical QMS solutions such as:
- When to open a deviation?
- Approaching a major non-conformance with sustainable solution?
- Use of HERCA in pharmaceutical: an uncommon method for better solution
- When validation is not validation and what are the better solutions
- Should you digitize your Quality Management System: pros and cons?
For pharmaceutical factory supervisors and staff, this seminar promises to be an engaging opportunity to gain novel insights. This is also an opportunity for students and university lecturers to gain a new understanding of quality systems.
WEBINAR INFO
- 8:30 a.m.–11:15 a.m., Sunday April 14, 2024
- Online via Zoom
Please feel free to contact SEN Pharma if you need any further information.