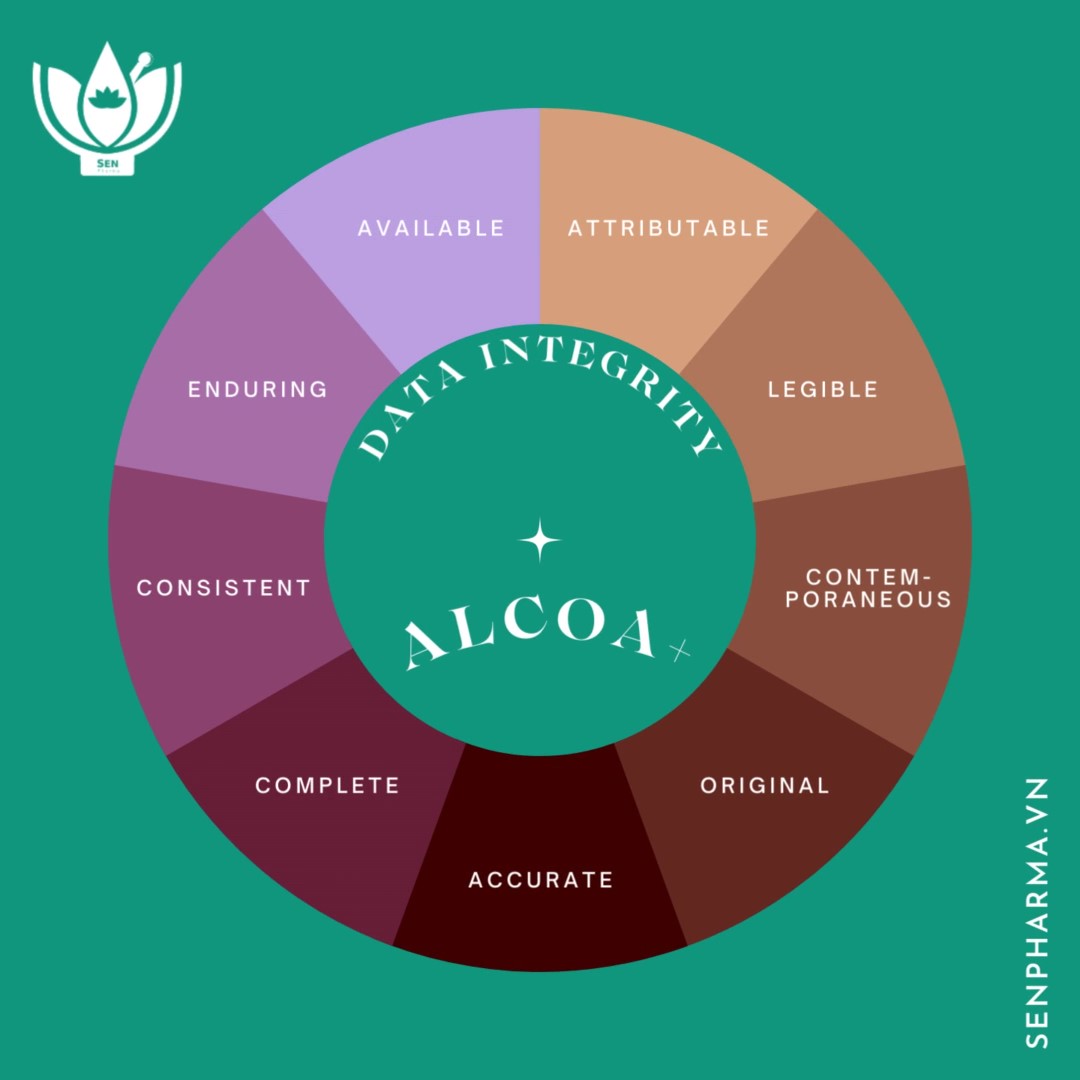
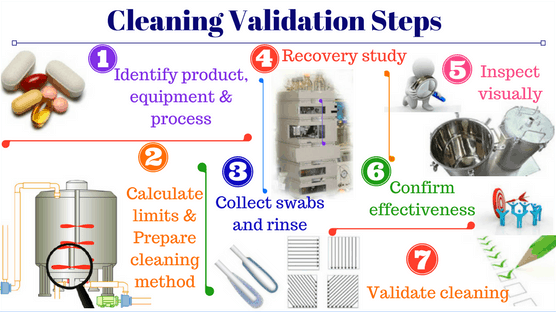
EU GMP is a standard of good manufacturing practice for medicines issued by the European Medicines Agency (EMA), covering all principles and standards to control activities or problems occurring in pharmaceutical manufacturing facilities in order to ensure the safety, efficacy, stability, and uniformity of each batch of products.
Advantages of EU GMP-compliant factories in Vietnam
With a large population of nearly 100 million people, along with economic development and increasing demand for healthcare, Vietnam is a potential market for high-quality pharmaceuticals.
According to SSI Research, only 6% of medications in Group 1 are produced domestically, while the majority of the remaining drugs are imported. To better compete with imported pharmaceuticals, products made in EU GMP-compliant facilities will be included in high-quality medicine Groups 1 and 2 in public hospital tenders.
Profits increase as exports expand into international markets.
Currently, Vietnam has approximately ten enterprises with production lines that fulfill EU-GMP standards, including a few big manufacturers in the southern region, such as:
- Dat Vi Phu Pharmaceutical JSC (Davipharm)
- Imexpharm Pharmaceutical Joint Stock Company
- Stellapharm J.V. Co., Ltd.
- Savi Pharmaceutical Joint Stock Company (Savipharm)
- Tenamyd Pharmaceutical Corporation
- Pymepharco Joint Stock Company
- Medochemie (Far East) Ltd., Co.
- Phil – Inter Pharma Co., Ltd
…
Let’s take a quick glance at the EU-GMP’s history and fundamentals in the following video from Lighthouse Worldwide Solutions.