Chuyên mục: Quality control
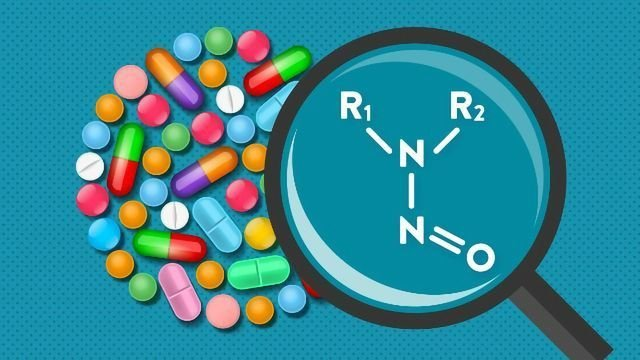
Nitrosamine in pharmaceuticals
What do we know about N-nitroso compounds (NOCs)? NOCs were first discovered about 40 years
Potential applications of Raman
Principle When you shine a laser on an atom, the photon will excite the electron