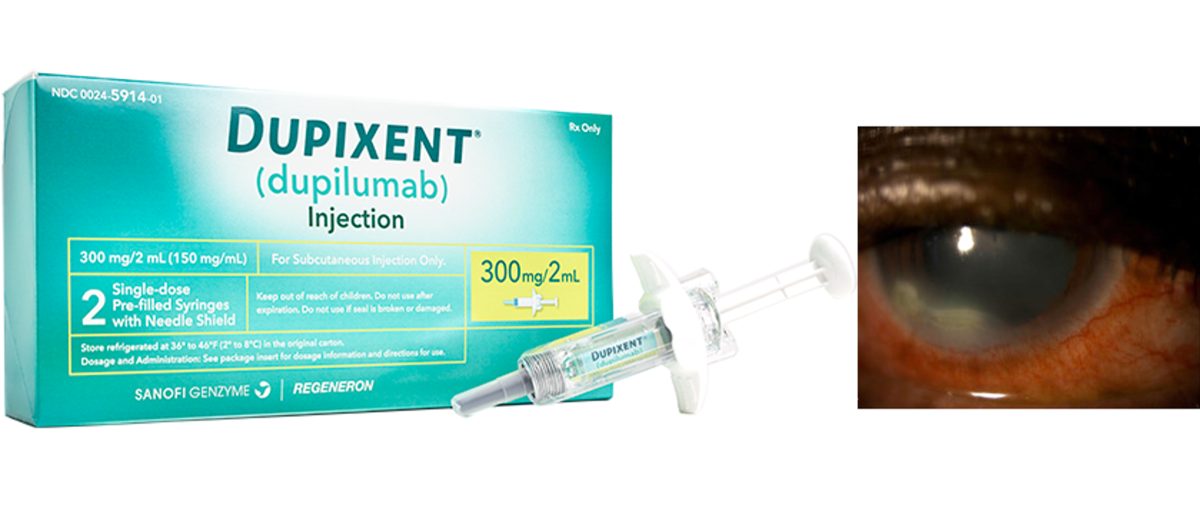
On November 29, 2022, the Medicines and Healthcare Products Regulatory Agency (MHRA) announced new serious eye-related side effects from Dupixent® that were detected in several UK patients.
Dupixent® (dupilumab) is known as an interleukin-4 and interleukin-13 inhibitor drug, which is usually prescribed for the treatment of atopic dermatitis, asthma, and nasal polyp.
In the past, physicians and patients were aware of the possibility of eye-related side effects such as conjunctivitis, keratitis, blepharitis, itchy eyes, etc., which were recorded in the guidelines. However, in clinical practice, these symptoms have mild impacts on patients and can be treated if properly managed.
Recently, the UK has submitted 479 reports to the MHRA of ocular adverse effects associated with Dupixent®, of which 111 were evaluated as serious conditions. Among these reports, there are nine reports of corneal ulceration, a symptom that directly affects the patient’s cornea and vision.
Despite several concerns about Dupixent®’s impact on patients’ quality of life, GlobalData still believes that Dupixent® will maintain its strong position in the allergic diseases market thanks to its effectiveness in treatment algorithms. In addition, the MHRA has published new guidance and product information for the management of patients with Dupixent®-related eye complications.
However, this could open up new opportunities for Dupixent®’s competitors, such as Adtralza (tralokinumab) by Leo Pharma, Lebrikizumab by Eli Lilly, or Neomolizumab by Galderma, if these products can prove their superiority in terms of safety and reduction of side effect frequency.
REFERENCES:
MHRA warns of serious eye-related adverse events after Dupixent use