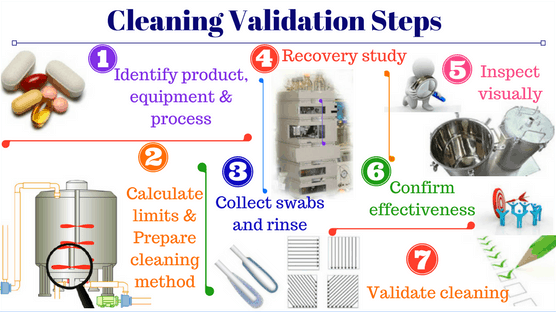
Medicine is of significance for human well-being, thus, the pharmaceutical production process must adhere strictly to hygiene standards to make sure pharmaceutical products will bring no harm to patients’ health. Cleaning validation is part of the manufacturing process to ensure that equipment and production processes are clean and free of microorganisms as well as residues.
Why is it needed?
Cleaning validation is proof that the cleaning process effectively removes residues of products and detergents to an acceptable extent and prevents microorganisms from developing.
What do we need to do for an effective cleaning validation procedure?
- Build a detailed cleaning validation protocol
- Develop a suitable sampling plan
- Pay attention to parts and locations that are difficult to clean
- Select the sampling locations with the highest risk to minimize the case of “false negatives”
- Select suitable products (products that are less likely to be dissolved)
- Validate at locations in direct contact with the product
- Validate after changing to another product
- Re-validate when there are risks or changes.
More importantly, the appraisers must develop a suitable and sufficient appraisal outline to evaluate the factory’s sanitation system.
What should an appraisal outline be?
- Target
- Subject
- Description of equipment used in the appraisal
- Time
- Personnel
- Sampling process
- Map of sampling location
- Sample preservation method
- Sample test time
- Proper cleaning procedures
- The control equipment needs to be used properly
Sampling methods
-
Indirect sampling for cleaning validation method (rinse sampling): A solvent like water is rinsed in a specific area of clean surface and tested for traces of contamination. This method is suitable for inaccessible systems or ones that can not be routinely disassembled.
-
Direct sampling for cleaning validation method (swab method): In this technique, a sterile material is systematically rubbed across the surface to be analyzed for the presence of residue.
REFERENCES: