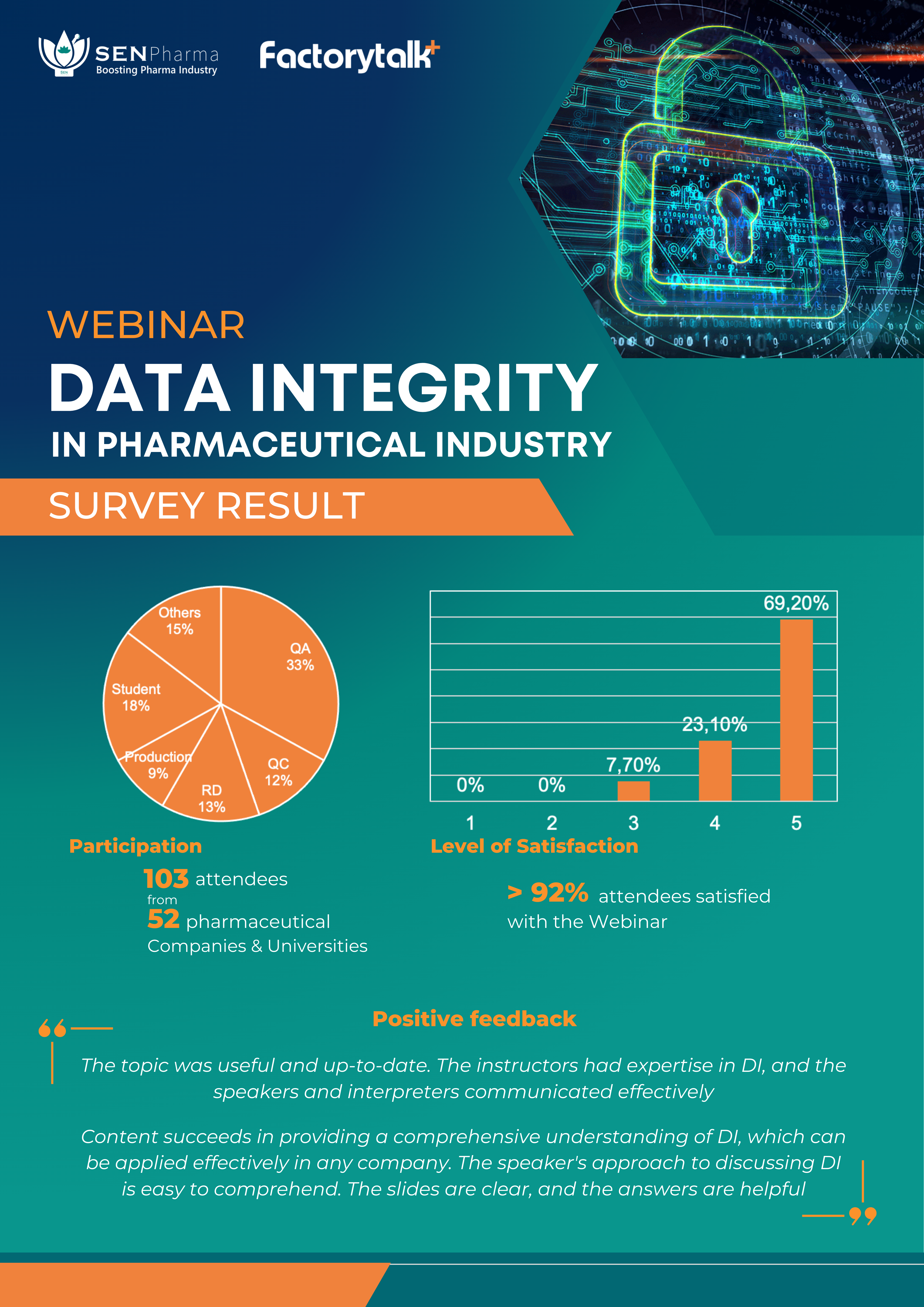
On Sunday, May 07, SEN Pharma and Factorytalk co-hosted a webinar titled “Data Integrity in the Pharmaceutical Industry” with great success. More than 100 customers from 52 organizations attended the event, the majority of which were pharma factories.
Ms. Manuh Pitasari, a Factorytalk specialist with over 15 years of expertise in GxP, computerized systems, and project management at pharmaceutical plants, provided a very thorough overview of data integrity (DI). During 2 hours and 30 minutes, the speaker emphasized the importance of Data Integrity in assuring product quality, patient health, and regulator trust. At pharma factories, particularly those looking to upgrade to EU-GMP, Data Integrity training should be considered one of the most important quality programs. The speaker not only presented definitions, regulations, and common misconceptions regarding Data integrity, but also analyzed short-term and long-term plans that can be implemented to improve Data Integrity.
✅ The first collaboration event between SEN Pharma and Factorytalk received very positive feedback from participants regarding the content, practicality, and usefulness of the training. This is a strong incentive for SEN Pharma and Factorytalk to continue arranging events to support pharma factories in the process of quality improvement, technological innovation, and internationalization.