Most solid-dose packaging still comes in bottles, but blister packaging is expanding globally. It is predicted that pharmaceutical blister packaging will undergo a compound annual growth rate of 5.1% to reach $8.9 billion by 2031.
Blister packaging is dominant in developing markets due to the advantage of making drugs more affordable and providing protection that can protect sensitive drugs well, even in extremely hot and humid conditions.
Another trend is focusing on patient-centricity. Patient-centered packaging offers an intuitive design and aids in helping consumers take their medications as prescribed, maximize compliance, ensure therapeutic efficacy and differentiate products in the market.
Packaging innovation
In response to sustainability and supply chain concerns, CDMO is expanding its packaging material options; adding or upgrading equipment, lines, and technology; and optimizing operations and supply chains.
1. In terms of materials, innovations include new materials, a protective barrier substrate, and a more sustainable substrate. For example, ACG Films and Foils has developed high-barrier polyvinylidene chloride (PVDC) films, more sustainable alternatives to PVC, and a cold-form five-layer aluminum foil.
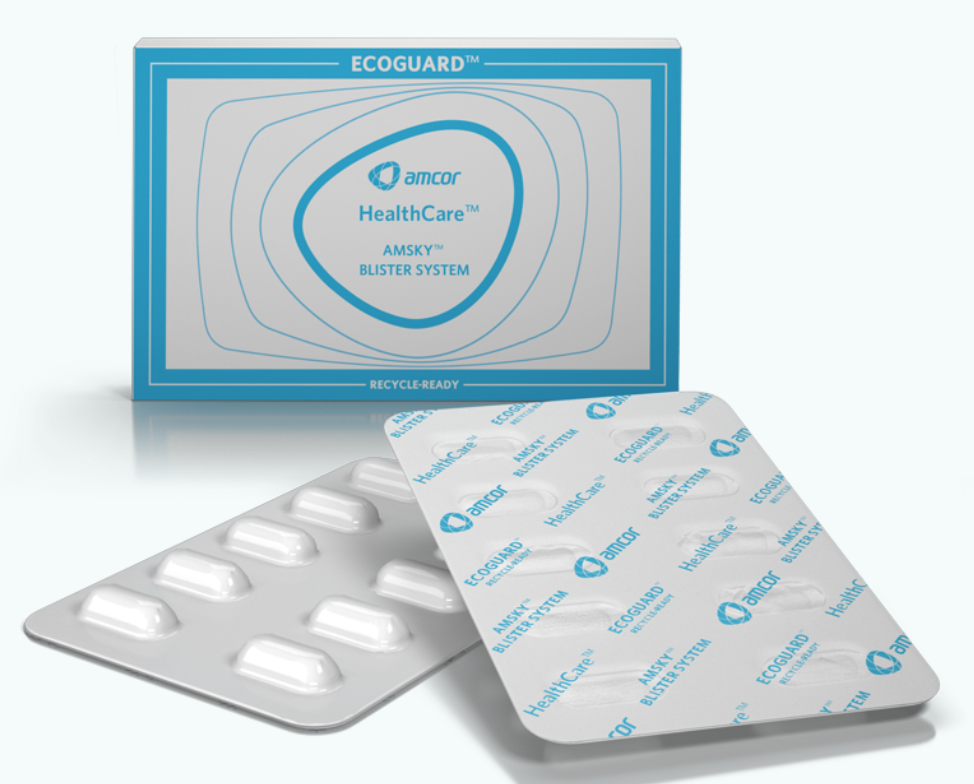
Another alternative to PVC is Amcor’s AmSky heating blister system. The printable material, based on polyethylene (PE), is compatible with PE recycling lines and contains no vinyl or aluminum foil. It is said that the barrier properties are equivalent to a 120g PVDC coating. The processing capacity is equal to existing materials, and the structure is child resistant.
2. Additionally, SÜDPACK Medica has created a coextruded polypropylene (PP) blister film that is more environmentally friendly to replace PVC/PVDC blister materials. The coextrusion technology can enhance barrier properties and close-loop material management.
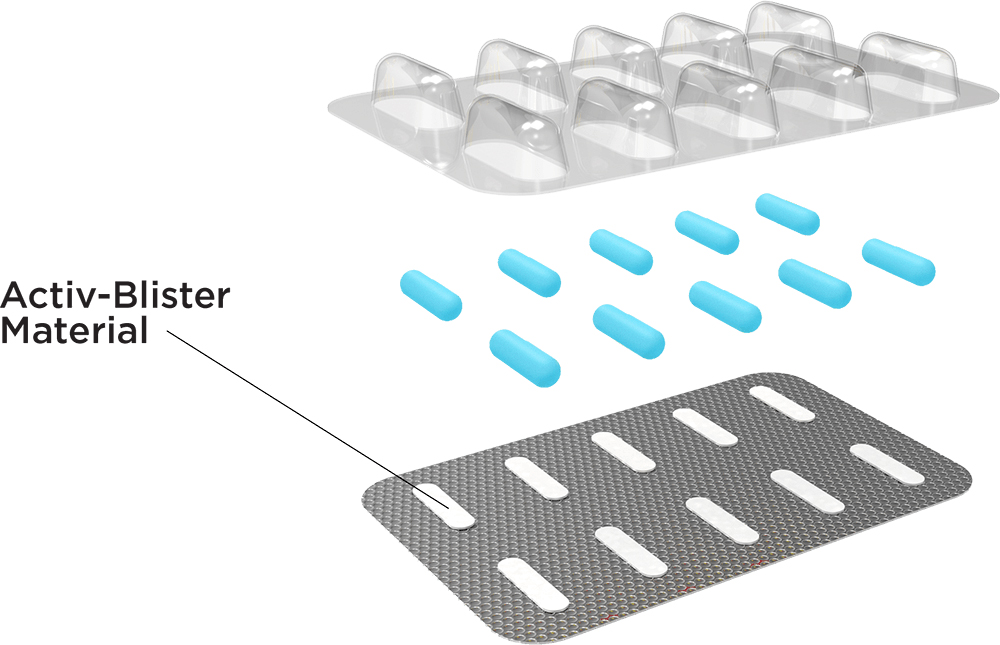
3. To increase the ability to protect products, Aptar CSP continues to develop its Activ-blister technology. The Activ-blister structure adds an engineered polymer membrane as a lid to control the humidity and oxygen levels in the voids of each blister chamber. “Activ-blister also helps to avoid complex reforming steps and extends the shelf-life of the solid oral products to withstand hard climatic conditions, such as zone 3 – hot and dry and zone 4 – hot and humid (according to ICH).
4. To avoid counterfeiting, Holographyx is working with Hazen Paper and Uhlmann Packaging to develop its Holo-blister technology. The Holo-blister technology allows conventional thermal-adhesive blister packers to cost-effectively apply a custom hologram to the back of the blister. Holo-Blister holograms are created to only be visible behind each pill or capsule cavity on the back of each blister pack, offering instant authenticity verification while leaving space around the recess to print marketing and dosage information.

What are the future trends in solid-dose packaging?
Future developments will focus on packaging sterilization, active packaging, sustainability, market flexibility, and patient-centric delivery to guarantee positive health outcomes and introduce a more sustainable approach for cradle-to-grave packaging lifecycles.
REFERENCES:
Meeting Challenges in Solid-Dose Packaging