The history of Chitosan
Chitosan is a macromolecule that has an amino polysaccharide structure. Chitosan is derived from Chitin, a biopolymer from marine sources, such as shrimps and crabs. Chitosan has been present in the pharmaceutical field since the early 1990s, and its potential soon attracted researchers to develop novel, effective modified-release dosage forms. Its polymer structure is composed of two monomers: N-acetyl-D-glucosamine and D-glucosamine. The presence of the amine group on the structure makes up for its name: amino polysaccharide. However, its poor solubility in both water and organic solvents makes the Chitosan delivery system rather hard to optimize the formulation.
The possibly first-ever scientific report of Chitosan dates back to 1859, as Charles Rouget obtained a product after boiling Chitin in concentrated potassium hydroxide. That fraction, surprisingly, is only soluble in aqueous acidic solutions, such as acetic acid, lactic acid, hydrochloric acid, and so on. Later, Chitosan was proved to be present in various organisms, mainly in fungi. However, its amount in nature compared to Chitin abundance is insignificant. Hence, most of the Chitosan is now obtained through the process of deacetylation of Chitin.
Structure of Chitosan
Chitosan’s units: N-acetyl-glucosamine and Glucosamine, grace Chitosan with their troublesome characteristic. The acetylated unit (N-acetyl-glucosamine) could form hydrogen bonds and exert hydrophobic interaction to stabilize the molecule. By contrast, the deacetylated unit (Glucosamine) could have its amino group ionized and become a cationic electrolyze. The presence of amino and hydroxyl moieties makes Chitosan a flexible structure, which could be modified to enhance its functionalities. These characteristics make Chitosan exert different interactions with different organic and inorganic solvents

Chitosan’s monomer structure is special that contributes to its advantages and disadvantages:
- The acetylation unit (N-acetyl-glucosamine) can form hydrogen bonds and contribute to the stability of the molecular structure.
- The deacetylated unit (glucosamine) contains an NH2 group that is easily ionized and positively charged to the polymer. Chitosan’s ability to have positive surface charge gives it an advantage in creating drug delivery vehicles compared to other biopolymers.
In addition, the presence of OH and NH2 groups in the structure helps Chitosan easily participate in chemical reactions to attach other groups of substances, making it compatible with each dosage form and each release purpose.
Despite its complex and difficult-to-modify nature, Chitosan also offers potential in creating perfect carriers.
Characteristic
- Solubility: poorly soluble in water, ethanol 95% , organic solvents and solutions pH > 6.5. Easily soluble in pH < 6.5. In different acid solutions, chitosan creates different viscosities.

- Moisture content: < 10%
- Chitosan is a cationic polyamine at pH < 6.5, so it is able to interact well with negatively charged agents and chelate complexes of metal ions.
- Chitosan contains nitrogen atoms so it is able to participate in amine group reactions such as N-acylation and Shciff reaction.
- Chitosan is stable at the room temperature but strongly hygroscopic after drying. Chitosan is incompatible with oxidizing agents.
- Physicochemical properties depend on: molecular weight, degree of deacetylation, environmental pH and charge distribution in the molecule.
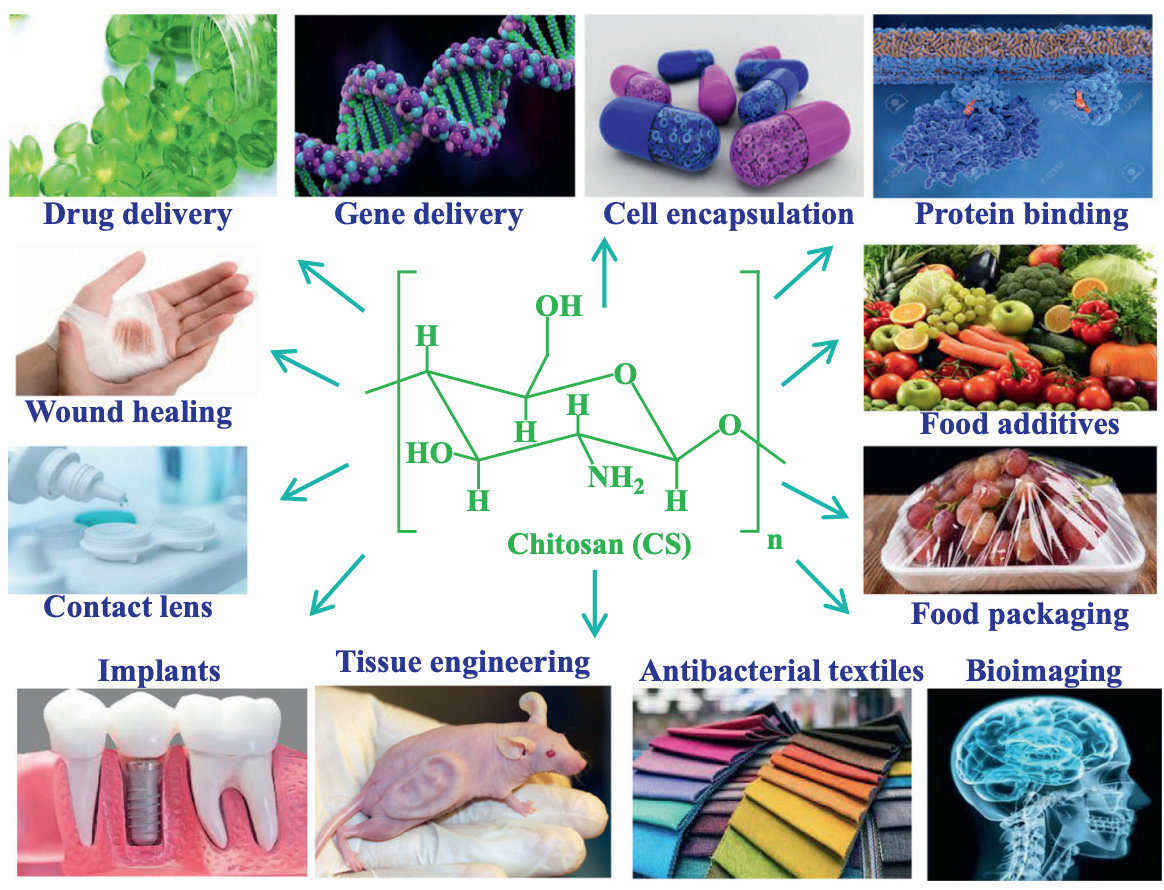
Application
The functional groups of Chitosan can adsorb various molecules, such as protein, lipids, metals, or even aromatic compounds or dyes, making it a possible vessel to carry different therapeutic agents to various sites of action. Chitosan, which was derived from natural sources, has good biodegradability and biocompatibility properties and could be applied to make cytocompatible drug carriers or effectively mimetic the extracellular matrix of the cytoskeleton in tissue engineering.
On the other hand, Chitosan bioactivity has been reported in various research, for instance, antimicrobial activity against diverse pathogens, including Gram-positive and Gram-negative bacteria. Antiviral effects are also mentioned, as Chitosan is thought to stop the replication process, inhibiting the viral infection.
Chitosan is not absorbed in the digestive tract, which makes it a possible dietary fiber. In several countries, it is listed as a food additive and is traditionally used in health-promoting foods such as dietary cookies, potato chips, and more.
Chitosan is researched to develop chitosan-based implant materials that have minimal effect on the human body. Besides its biodegradable, biocompatibility, and adsorption capacity properties, Chitosan is reported to generate several physiological effects, such as hemostasis and macrophage activation in inflammation. It is further indicated that the stimulant of Chitosan may have a positive effect on cell growth, viability, and proliferation.
The distinguished functional properties of Chitosan make it a potential material for developing novel therapeutic systems. Even though having been used for a long time, Chitosan keeps opening the possibility for a diversity of new applications in all bio-friendly industries in general, specifically the pharmaceutical industry.
REFERENCES:
- Chapter 1 – Chemical Characteristics and Functional Properties of Chitosan, Chitosan in the Preservation of Agricultural Commodities, 2016.
- Chitosan: A review of sources and preparation methods, International Journal of Biological Macromolecules.
- A review on chitosan and its nanocomposites in drug delivery, International Journal of Biological Macromolecules.
- Pharmaceutical applications of chitosan