In pharmaceutical manufacturing, water plays a crucial role. Water can be a solvent in the processing, an ingredient, or one part of the finished product. Water also used as a cleaning agent for rinsing tools, equipment, rooms, etc. For individual applications, water will be treated differently and has its standards..
Quality standards of water are specified in monographs of pharmacopeia. Each grade is used for a specific stage of manufacturing or product. The European Pharmacopoeia provides quality standards for the following grades of water:
- Water for preparation of extracts
- Water for injection (WFI): used in the preparation of parenteral administration, peritoneal dialysis solutions,… and cleaning of parts in direct contact with the product (for sterile preparations)
- Purified water: used in the production of non-injectable preparations (oral tablets, preparations for external use, eye/nasal/ear drops, etc.), equipment cleaning process (non-parenteral product-contact components), tests, and analyses are required.
Potable water is not covered by a monograph but must comply with requirements defined in Directive 98/83/EC. Potable water often is used in the initial steps of chemical synthesis or equipment cleaning unless there are specific technical or quality requirements.
The following criteria are commonly used to test water quality:
- Appearance
- pH
- Conductivity
- Total dissolved solids (TDS)
- Total organic carbon (TOC)
- Nitrates
- Heavy metal
- Microbiological limit
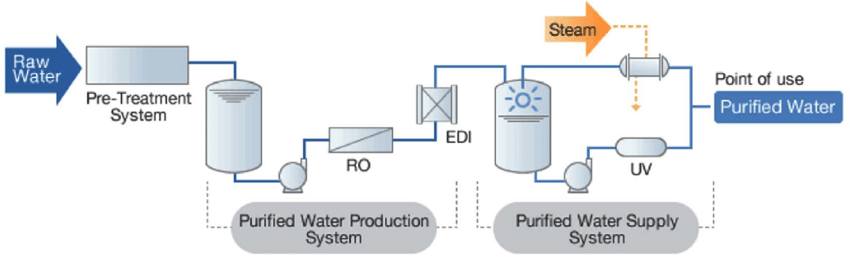
Purified water used in pharmaceuticals needs to be treated before reaching the downstream unit to minimize chemical and microbiological contaminants. The water supply for the treatment process can be obtained from a potable water system. There are various methods used in the water treatment process:
- Pre-treatment stage usually uses filtration methods: ultrafiltration, microfiltration, and multimedia filter.
- RO filter system (reverse osmosis). This system contains a semi-permeable membrane that allows passing water and rejects the contaminants such as particles, ions, microorganisms
- Deionization: ion exchange, continuous electrolysis, distillation, reverse osmosis.
- TOC reduction process: using activated carbon, ozone, reverse osmosis, distillation
- Microbiological control: using disinfectants, ultraviolet light, ozone, reverse osmosis, distillation, and ultrafiltration.
Water treatment systems should be designed, installed, and qualified to achieve the required levels based on pharmacopeia or GMP requirements. In addition, it is important to have quality control strategies such as risk assessment for potential contamination, design of operating procedures, periodic testing, cleaning, maintenance, and control of related changes.
REFERENCES:
- Guideline on the quality of water for pharmaceutical use of European Medicines Agency
- QUALITY OF WATER FOR PHARMACEUTICAL USE: AN OVERVIEW
- Purified water system: Biocontamination control techniques in pharmaceuticals




